Tryptamine
Tryptamine sind chemische Verbindungen, die vom 2-(Indol-3-yl)ethylamin abgeleitet sind. Sie sind Stoffwechselprodukte zahlreicher Lebewesen (vor allem Pflanzen) und zählen zu den Indolalkaloiden. Prominente Derivate mit Tryptamin-Struktur sind die Neurotransmitter Serotonin und Melatonin, die Aminosäure Tryptophan und die psychedelisch wirksamen Halluzinogene Dimethyltryptamin und Psilocybin sowie Psilocin.
Die wesentlichen Strukturmerkmale von Tryptaminen und Phenylethylaminen finden sich in den Lysergsäureamiden vereint.[1]
Tabellarische Übersicht
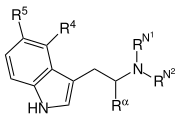 | |||||||
|---|---|---|---|---|---|---|---|
| Name | R4 | R5 | Rα | RN1 | RN2 | chemischer Name | Herkunft |
| Tryptamin | H | H | H | H | H | 2-(Indol-3-yl)ethylamin | natürlich |
| N-Methyltryptamin (NMT) | H | H | H | H | CH3 | N-Methyltryptamin | natürlich |
| 5-Methoxy-N-methyltryptamin (5-MeO-NMT) | H | OCH3 | H | H | CH3 | 5-Methoxy-N-methyltryptamin | natürlich |
| Dimethyltryptamin | H | H | H | CH3 | CH3 | N,N-Dimethyltryptamin | natürlich |
| O-Methylbufotenin (5-MeO-DMT) | H | OCH3 | H | CH3 | CH3 | 5-Methoxy-N,N-dimethyltryptamin | natürlich |
| 5-Brom-N,N-dimethyltryptamin (5-Brom-DMT) | H | Br | H | CH3 | CH3 | 5-Brom-N,N-dimethyltryptamin | natürlich |
| Bufotenin | H | OH | H | CH3 | CH3 | 5-Hydroxy-N,N-dimethyltryptamin | natürlich |
| N-Methylserotonin | H | OH | H | CH3 | H | 5-Hydroxy-N-methyltryptamin | natürlich |
| Serotonin | H | OH | H | H | H | 5-Hydroxytryptamin | natürlich |
| Psilocin | OH | H | H | CH3 | CH3 | 4-Hydroxy-N,N-dimethyltryptamin | natürlich |
| Psilocybin | OPO3H2 | H | H | CH3 | CH3 | 4-Phosphoryloxy-N,N-dimethyltryptamin | natürlich |
| Baeocystin | OPO3H2 | H | H | CH3 | H | 4-Phosphoryloxy-N-methyltryptamin | natürlich |
| Norbaeocystin | OPO3H2 | H | H | H | H | 4-Phosphoryloxytryptamin | natürlich |
| Melatonin | H | OCH3 | H | O=C-CH3 | H | 5-Methoxy-N-acetyltryptamin | natürlich |
| Tryptophan | H | H | COOH | H | H | α-Carboxytryptamin | natürlich |
 | |||||||
| Name | R4 | R5 | Rα | RN1 | RN2 | chemischer Name | Herkunft |
| Alpha-Methyltryptamin (AMT) | H | H | CH3 | H | H | α-Methyltryptamin | synthetisch |
| 5-Methoxy-α-methyltryptamin (5-MeO-AMT) | H | OCH3 | CH3 | H | H | 5-Methoxy-α-methyltryptamin | synthetisch |
| α-Ethyltryptamin (AET) | H | H | CH2CH3 | H | H | α-Ethyltryptamin | synthetisch |
| N-Ethyltryptamin (NET) | H | H | H | H | CH2CH3 | N-Ethyltryptamin | synthetisch |
| Diethyltryptamin (DET) | H | H | H | CH2CH3 | CH2CH3 | N,N-Diethyltryptamin | synthetisch |
| Diisopropyltryptamin (DiPT) | H | H | H | CH(CH3)2 | CH(CH3)2 | N,N-Diisopropyltryptamin | synthetisch |
| Dipropyltryptamin (DPT) | H | H | H | CH2CH2CH3 | CH2CH2CH3 | N,N-Dipropyltryptamin | synthetisch |
| Dibutyltryptamin (DBT) | H | H | H | CH2CH2CH2CH3 | CH2CH2CH2CH3 | N,N-Dibutyltryptamin | synthetisch |
| 4-Hydroxy-N-methyl-N-ethyltryptamin (4-HO-MET) | OH | H | H | CH3 | CH2CH3 | 4-Hydroxy-N-methyl-N-ethyltryptamin | synthetisch |
| 4-Hydroxy-N,N-diethyltryptamin (4-HO-DET) | OH | H | H | CH2CH3 | CH2CH3 | 4-Hydroxy-N,N-diethyltryptamin | synthetisch |
| 4-Phosphoryloxy-N,N-diethyltryptamin (4-PO-DET) | OPO3H2 | H | H | CH2CH3 | CH2CH3 | 4-Phosphoryloxy-N,N-diethyltryptamin | synthetisch |
| 4-Hydroxy-N,N-diisopropyltryptamin (4-HO-DIPT) | OH | H | H | CH(CH3)2 | CH(CH3)2 | 4-Hydroxy-N,N-diisopropyltryptamin | synthetisch |
| 4-Hydroxy-N-isopropyl-N-methyltryptamin (4-HO-MiPT) | OH | H | H | CH(CH3)2 | CH3 | 4-Hydroxy-N-isopropyl-N-methyltryptamin | synthetisch |
| 5-Methoxy-N,N-diallyltryptamin (5-MeO-DALT) | H | OCH3 | H | H2C=CH-CH2 | H2C=CH-CH2 | 5-Methoxy-N,N-diallyltryptamin | synthetisch |
| 5-Methoxy-N,N-diisopropyltryptamin (5-MeO-DiPT) | H | OCH3 | H | CH(CH3)2 | CH(CH3)2 | 5-Methoxy-N,N-diisopropyltryptamin | synthetisch |
| 5-Methoxy-N,N-methylisopropyltryptamin (5-MeO-MiPT) | H | OCH3 | H | CH3 | CH(CH3)2 | 5-Methoxy-N,N-methylisopropyltryptamin | synthetisch |
| Sumatriptan | H | CH2SO2NHCH3 | H | CH3 | CH3 | 5-Methylaminosulfonyl-N,N-dimethyltryptamin | synthetisch |
Synthese
Tryptamin kann durch das Abramovitch-Shapiro-Syntheseverfahren hergestellt werden.[2]

Abramovitch-Shapiro-Syntheseverfahren
Grafische Übersicht
Übersicht über eine kleine Auswahl an endogenen, natürlichen und synthetischen Tryptaminen.
Körpereigene, pflanzliche und synthetische Tryptamine
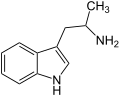 α-Methyltryptamin
α-Methyltryptamin α-Ethyltryptamin
α-Ethyltryptamin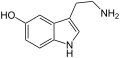 Serotonin (5-Hydroxytryptamin)
Serotonin (5-Hydroxytryptamin)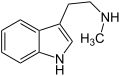 N-Methyltryptamin
N-Methyltryptamin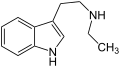 N-Ethyltryptamin
N-Ethyltryptamin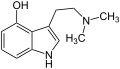 Psilocin
Psilocin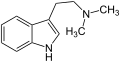 Dimethyltryptamin (N,N-Dimethyltryptamin)
Dimethyltryptamin (N,N-Dimethyltryptamin)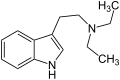 Diethyltryptamin (N,N-Diethyltryptamin)
Diethyltryptamin (N,N-Diethyltryptamin) L-Tryptophan (natürliche Aminosäure)
L-Tryptophan (natürliche Aminosäure)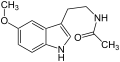 Melatonin
Melatonin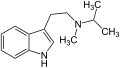 MiPT
MiPT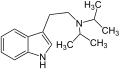 DiPT
DiPT
Literatur
- Alexander Shulgin, Ann Shulgin: TiHKAL: The Continuation. Transform Press, Berkeley 1997, ISBN 0-9630096-9-9.
- Keeper of the Trout & Friends: Some Simple Tryptamines (Second Edition). Mydriatic Productions, 2007, ISBN 978-0-9770876-5-5.
- Ana Margarida Araújo, Félix Carvalho u. a.: The hallucinogenic world of tryptamines: an updated review. In: Archives of Toxicology. 89, 2015, S. 1151, doi:10.1007/s00204-015-1513-x.
Einzelnachweise
- Fabrizio Schifano, Laura Orsolini, Duccio Papanti, John Corkery: NPS: Medical Consequences Associated with Their Intake. In: Michael H. Baumann, Richard A. Glennon, Jenny L. Wiley: Neuropharmacology of New Psychoactive Substances (NPS). Springer, E-Book 2016, ISBN 978-3-319-52444-3, S. 364.
- R. A. Abramovitch, D. Shapiro: 880. Tryptamines, carbolines, and related compounds. Part II. A convenient synthesis of tryptamines and β-carbolines. In: Journal of the Chemical Society (Resumed). 1956, S. 4589, doi:10.1039/JR9560004589.
This article is issued from Wikipedia. The text is licensed under Creative Commons - Attribution - Sharealike. Additional terms may apply for the media files.